https://www.odinhealthtech.com/#contact
< home > 7-9-2021
| 01 | BROADCAST MEDIA ( HOLD UNTIL UNSATISFACTORY GOVERNMENT RESPONSE IS APPARENT ) | STATUS: (ASSUME not DONE - UNTIL "done" APPEARS | |
|
BROADCAST MEDIA - "INVESTIGATIVE" REPORTING ( https://www.ire.org/contact/ ) |
|||
| 02 | Politicians: My Congressman, BROWN 01, pORTMAN 02, US President: BIDEN | ||
| 03 | MEDICAL DEVICE (MRI RELATED) MANUFACTURERS - Email version | ||
|
sales-dept@mizuhomedical.co.jp (DONE : surgical supply manufacturer NOT MRI MACHINE ) |
|||
| 04 | MAGNETIC RESONANCE DEVICE MANUFACTURER - CONTACT: FORM VERSION | ||
| https://www.odinhealthtech.com/#contact https://www.imris.com/contact/ https://www.oncologysystems.com/contact-us https://www.esaote.com/contacts/ https://us.medical.canon/contact/ https://www.usa.philips.com/healthcare/medical-specialties/covid-19#_form https://www.siemens-healthineers.com/en-us/how-can-we-help-you https://www.gehealthcare.com/about/contact-us |
|||
| 05 | MEDICAL Safety TRAINING - PROFESSIONALS (MRI SPECIFIC) | ||
| frank.shellock@mrisafety.com | |||
| 06 | MEDICAL Professors & Teachers | ||
| tkachjn@ucmail.uc.edu david.hintenlang@osumc.edu ugurb001@umn.edu rmenon@robarts.ca bhinfo@uw.edu |
|||
| 07 | MEDICAL Publishers | ||
| AJNR.EIC@gmail.com support@ispub.com |
|||
| 08 |
Research ScientistS - America Research Facility - FRANCE |
||
| 09 | Academic Medical Centers | ||
| ivan.pedrosa@utsouthwestern.edu robert.lenkinski@utsouthwestern.edu neil.rofsky@utsouthwestern.edu Ltsai1@bidmc.harvard.edu akgrant@bidmc.harvard.edu kmortele@bidmc.harvard.edu jkung@bidmc.harvard.edu shagen@bidmc.harvard.edu |
|||
| 10 | Hospital - Technology Sites | ||
| drconnect@ccf.org medicalconcierge@ccf.org mvhssurgeryadministration@PremierHealth.com :: (DONE EMAILED) [ MARY Boosalis - PREMIER HEALTH-6-5-2021-PDF-VERSION.pdf :: cONTACT FORM ] rearoberts@premierhealth.com (DONE EMAILED) hrkirkpatr@PremierHealth.com (DONE EMAILED) |
|||
| 11 | US-FDA | ||
| jana.delfino@fda.hhs.gov ting.song@fda.hhs.gov sunder.rajan@fda.hhs.gov shingchunbenny.lam@fda.hhs.gov wolfgang.kainz@fda.hhs.gov Daniel.Krainak@fda.hhs.gov combination@fda.gov FDAImportsInquiry@fda.hhs.gov DICE@fda.hhs.gov reglist@cdrh.fda.gov device.reg@fda.hhs.gov |
|||
| 12 | CDRH Management - HHS/fda | ||
| jeff.shuren@fda.hhs.gov Douglas.KellyMD@fda.hhs.gov Ellen.Flannery@fda.hhs.gov Elizabeth.Hillebrenner@fda.hhs.gov Melissa.Torres@fda.hhs.gov Nicole.Ellis@fda.hhs.gov Nancy.Braier@fda.hhs.gov Olga.Claudio@fda.hhs.gov Cathy.Oliveri@fda.hhs.gov Jerry.Menikoff@hhs.gov >>>>> done Julia.Gorey@hhs.gov douglas.diekema@seattlechildrens.org ocrmail@hhs.gov OCRMedia@hhs.gov [ PDF VERSION OF REQUEST ] via Jerry.Menikoff@hhs.gov (done) |
|||
| 13 | Center for Biologics Evaluation and Research -- ALSO REFERRED TO AS: CBER | ||
| Peter.Marks@fda.hhs.gov < DIRECTOR email@asn-online.org < Witten, Celia ASSISTANT DIRECTOR khi@asn-online.org < Witten, Celia ASSISTANT DIRECTOR cberombudsman@fda.hhs.gov |
|||
| 14 | Streamline Regulatory Support Service (example) | ||
| stephen.rhodes@streamlineregulatory.com |
"titanium" "aneurysm" "clip" < Google
https://www.aesculapusa.com › or-solutions › aneurysm-cl...
Titanium and Phynox Aneurysm Clips · Available in standard and fenestrated styles · Color coded with gold or silver blades (temporary or permanent clip) · Low ...
How long do aneurysm clips last?
Results—The mean interval from surgery was 9.3 years for all patients and 9.0 years for the clipped aneurysms (range 3 to 21 years).May 1, 2001
https://www.ahajournals.org › abs › 01.STR.32.5.1191
Search for: How long do aneurysm clips last?
Are aneurysm clips metal?
Aneurysm clips come in a wide variety of shapes and blade lengths and are made from different materials with varying magnetic susceptibilities. ... Materials used to make these aneurysm clips included stainless steel alloy, Phynox, Elgiloy, commercially pure titanium, and titanium alloy.Mar 1, 2003
Search for: Are aneurysm clips metal?
Are titanium clips MRI safe?
Titanium is a paramagnetic material that is not affected by the magnetic field of MRI. The risk of implant-based complications is very low, and MRI can be safely used in patients with implants.
https://www.ncbi.nlm.nih.gov › articles › PMC6369045
Search for: Are titanium clips MRI safe?
What are brain aneurysm clips made of?
Clips are made of titanium and remain on the artery permanently. Figure 1. Most aneurysms resemble a balloon, with a narrow neck at its origin and a large expanding dome. During surgery, a clip is placed across the neck of the aneurysm to prevent blood from entering.
https://mayfieldclinic.com › pe-clipping
Search for: What are brain aneurysm clips made of?
Are all Sugita aneurysm clips MRI safe?
Sugita clips have been shown to be acceptable for use with MRI systems, of up to 1.5 Tesla, presently in clinical use.
The jaw profile, consisting of a pyramid-shaped inner surface design, helps to prevent clip slippage and evenly distributes the closing pressure throughout the full length of the jaw.
http://www.kebomed.dk › sugita_elgiloy_clips_brochure
Search for: Are all Sugita aneurysm clips MRI safe? < LOOK AT THIS LINK!
What happens after an aneurysm clipping?
You will probably feel very tired for several weeks after this surgery. You may also have headaches or problems concentrating for 1 to 2 weeks. It can take 4 to 8 weeks to fully recover. The incisions may be sore for about 5 days after surgery.
https://myhealth.alberta.ca › Health › pages › conditions
Search for: What happens after an aneurysm clipping?
Can an aneurysm clip come loose?
There is a very low likelihood they will come loose or that the aneurysm will come back, if the clips were placed satisfactorily at the time of your operation.Feb 12, 2009
https://thechart.blogs.cnn.com › 2009/02/12 › how-long-...
Search for: Can an aneurysm clip come loose?
Is aneurysm clipping risky?
Risks of aneurysm clipping include bleeding, infection, and stroke-like symptoms.
https://www.urmc.rochester.edu › neurosurgery › services
Search for: Is aneurysm clipping risky?
Can you fully recover from an aneurysm?
It will take 3 to 6 weeks to fully recover. If you had bleeding from your aneurysm this may take longer. You may feel tired for up to 12 or more weeks. If you had a stroke or brain injury from the bleeding, you may have permanent problems such as trouble with speech or thinking, muscle weakness, or numbness.Jul 20, 2020
https://medlineplus.gov › ency › patientinstructions
Search for: Can you fully recover from an aneurysm?
What are the chances of surviving aneurysm surgery?
Surgeons and hospitals have no central board accrediting them on their performance of aneurysm surgery, nor are they required to publish their own track record in this area. Studies in medical journals suggest that the death rate ranges from zero to 7%, and the complication rate from 4% to 15%.Oct 30, 2000
https://www.webmd.com › brain › news › brain-aneurysm...
Search for: What are the chances of surviving aneurysm surgery?
How do they clip an aneurysm?
During microsurgical clipping, a small metal clip is used to stop blood flow into the aneurysm. A craniotomy is performed to create an opening in the skull to reach the aneurysm in the brain. The clip is placed on the neck (opening) of the aneurysm to obstruct the flow of blood, and remains inside the brain.
https://www.hopkinsmedicine.org › aneurysm_clipping
Search for: How do they clip an aneurysm?
What size aneurysm requires surgery?
the size of the aneurysm – aneurysms larger than 7mm often require surgical treatment, as do aneurysms larger than 3mm in cases where there are other risk factors. the location of the aneurysm – brain aneurysms located on larger blood vessels have a higher risk of rupture.
https://www.nhs.uk › conditions › treatment
Search for: What size aneurysm requires surgery?
What is a titanium clip?
If you've had a surgical biopsy on your breast (or you're about to get one), you may know that your doctor uses a tiny titanium clip (the size of a sesame seed) to mark the spot that's been tested for cancer. This identifies the area if you ever need further intervention.Jul 16, 2020
https://www.healthcentral.com › slideshow › titanium-mar...
Search for: What is a titanium clip?
What metal is not allowed in MRI?
Therefore, if you have to do repairs or otherwise conduct work in an MRI environment, you must use MRI-compatible products. Any tools and blades you use can't be magnetic. Since these objects are most commonly made of, or contain, steel, which is definitely magnetic, they're a no-go.
https://blog.sliceproducts.com › mri-compatible
Search for: What metal is not allowed in MRI?
Are surgical clips safe?
For the most part, surgical clips are not a problem because modern clips aren't ferromagnetic. The exception is surgical clips used to repair a brain aneurysm. These can be dangerous, says Dr.Feb 28, 2017
https://www.yalemedicine.org › news › mri-safety
Search for: Are surgical clips safe?
How long should surgical clips stay in?
Are clips MRI safe?
Why are surgical clips left in?
Will a magnet stick to titanium?
What happens if you go into MRI with metal?
What is alternative to MRI?
Do surgical clips dissolve?
Why do they put titanium in breast?
How long does Titanium stay in system?
Are there warning signs before an aneurysm?
How serious is a 5 mm aneurysm?
Should I worry about an aneurysm?
What is the difference between an embolism and an aneurysm?
Which is better coiling or clipping?
What is an advantage of early clipping of cerebral aneurysm?
What triggers an aneurysm?
How can you prevent an aneurysm from rupturing?
How do you stop an aneurysm from growing?
Does a brain aneurysm shorten your life?
What should you do after an aneurysm?
Can an unruptured aneurysm cause personality changes?
What is the disadvantage of early clipping of cerebral aneurysm?
What are the chances of having a second aneurysm?
How long is hospital stay after brain aneurysm surgery?
Can someone live with a brain aneurysm?
How long does an aneurysm operation take?
Can you drive after an aneurysm?
Can you fly with aneurysm?
Can a brain aneurysm come back after surgery?
What should you not do before an MRI?
What are the three types of aneurysms?
https://www.aesculapusa.com › or-soultions › pdfs
Non-clinical testing demonstrated that the Yasargil Aneurysm Clip is MR Conditional. A patient with this device can be safely scanned immediately after ...
1 page
https://pubmed.ncbi.nlm.nih.gov › ...
by MT Lawton · 1996 · Cited by 56 — A new aneurysm clip of pure titanium was developed to minimize artifact on postoperative MR images. We evaluated these clips in a series of mechanical tests ...
http://www.mrisafety.com › SafetyInformation_view
The presence of an aneurysm clip in a patient referred for an MR procedure ... Aneurysm clips made from commercially pure titanium (Spetzler), Elgiloy (Sugita), ...
http://www.mizuhomedical.co.jp › products
Sugita Titanium Aneurysm Clip II (T2) & Appliers|Aneurysm Clips|Mizuho provides effective solutions for surgical fields with our products of reliability.
by FG Shellock · 2003 · Cited by 46 — Each aneurysm clip was qualitatively evaluated for magnetic field ... Aneurysm clips made from commercially pure titanium and titanium alloy ...
Jun 1, 2021 — 4, Spetzler pure titanium aneurysm clip, model C-2200; straight, 5-mm blade; C.P. titanium; NMT Neurosciences, Duluth, Georgia, Negative, 0 ...
https://thejns.org › journals › j-neurosurg › article-p1260
by Y Otawara · 2010 · Cited by 8 — The characteristics of a Yasargil titanium aneurysm clip were evaluated after long-term implantation for 12 years in a patient with a cerebral ...
https://surgicalone.com › Aneurysm Clips
A Clip for Every Need To facilitate treatment of complex aneurysms, the T2 Clip is available in a wide variety of blade shapes, lengths and angles. Clip-Spring ...
https://radiology.ucsf.edu › files › patient-safety
Sugita T2 aneurysm Clip, Yasargil Phynox aneurysm clip (FE),. Yasargil Titanium aneurysm clip (FT)). • Aneurysm clips places at an outside hospital, must have ...
19 pages
yasargil aneurysm clip mri safety
aneurysm clip mri compatibility
sugita titanium aneurysm clip mri safety
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Next |
45405, Dayton, OH - Based on your past activity - Use precise location - Learn more
MEDICAL DEVICE (MRI RELATED) MANUFACTURERS
MEDICAL DEVICE MANUFACTURER - CONTACT:Email
sales-dept@mizuhomedical.co.jp < done [ PDF VERSION OF REQUEST ]
healthcare@hitachi.co.in
brian.liuba@us.neusoft.com
info@fonar.com
customersupport@imris.com
customerservice@mizuho.com
porous polyethylene surgical devices.
customercare@poriferous.com
MEDICAL DEVICE MANUFACTURER - CONTACT: form format (Manufacturer DRAFT MESSAGE EXAMPLE & MODEL: )
Dear Magnetic Resonance Imaging ( "Medical Device") manufacturer, my name is Susan Neuhart.
-- As a United States citizen - I believe, your "product" represents a "clear and present danger" - to "me"
- AND all Americans "similarly situated" to me - as I describe below [ cites A , B ].
Thus, I will need to see the documents you submitted - to the US FDA - related to product "verifications and experiments" YOU performed and submitted - WHICH MISLED THE US FDA.
Please consult FDA document [Cite C] - to see the basis of my initial request - AND - what I am anticipating to receive - from your agent.
All parties will appreciate your "anticipation" of my needs - AND, ATTEMPTS to "obfuscate" - WILL NOT BE TOLERATED.
I describe my circumstances - in detail - in the documents [that] I have submitted to the US FDA/HHS [ cites A , B ]
You can review my qualifications - to review the documents [YOU tender (to me) ] here > [ https://hansandcassady.org/ABOUTSusanResume.html ]
I prefer you cite URLs [that] I (and other interested parties) may access easily; And, you should anticipate [that] I will post our correspondence - by Email - to my personal web site: ( https://hansandcassady.org/ )
I DO NOT SELL ANYTHING ON MY PERSONAL WEB SITE. YOU MAY USE MY EMAIL [THAT] I HAVE SUPPLIED ON YOUR CONTACT FORM.
As a graduate of the University of Wisconsin, I have a preferred Scientific Report format [ https://writing.wisc.edu/handbook/assignments/sciencereport/ ]
I can accommodate slight differences - but, a "Science Report" does contain certain basic information - which, I will expect. We (all parties) must make a "determination" - if, you have collected additional data (previously unpublished).
YOU should anticipate [that] I will politely ask your designated AGENT "follow up" questions - and, please supply relevant information. THAT IS:
Your Agent's Name, Title, Employer (WITH ORGANIZATIONAL "REPORT TO" SPECIFIED), Qualifications, Email, etc. I will redact their Email address (in published documents) - if requested). I am fluent in "American English" only.
Time is of the essence! - as I relate here [ cites A , B ]
Please consult FDA document [cite C] : to see the basis of my request - AND - what I am anticipating to receive - from your agent - at the start.
All parties will appreciate your "anticipation" of my needs - AND, ATTEMPTS TO "obsfuscate" - WILL NOT BE TOLERATED.
Again "Time is of the essence" - as I relate here [ cites A , B ]. Sincerely - Susan
Cites:
A - https://hansandcassady.org/Email-HHS-Secretary%20-PDF-VERSION-6-3-2021-final-6-3-2021-FINAL.pdf < Xavier Becerra US Secretary HHS
B - https://hansandcassady.org/FDA-CDRH-CBER-HHS-version-6-7-2021-pdf-VERSION.pdf < Message tailored for FDA, CDRH, CBER
C - [ https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=807&showFR=1&subpartNode=21:8.0.1.1.5.5
( ( ... TITLE 21-USA-FDA-HHS SUBCHAPTER H - MEDICAL DEVICES - PART 807... ) ]
SOURCE: http://library.unisel.edu.my/equip-unisel/custom/ebook_catalog/ebook/RegulatoryAffairsforbiomaterialsandMedicalDevices.pdf ( https://www.unisel.edu.my/contact/ ) [ EDIT BY SUSAN ]
( Jalan Timur Tambahan 45600 Bestari Jaya Selangor Darul Ehsan Malaysia : GOOGLE MAP )
"... 2.8 Understanding and complying with FDA guidelines ... There is a lot of confusion regarding what data need to be provided with any given [MEDICAL DEVICE] submission.
[ SUSAN NEUHART WOULD ELIMINATE THIS! ]
... In order to help the process of "commercialization", the FDA has issued many guidance documents.
... By design, the guidance documents are not prescriptive in nature, but talk about the kinds of issues that need to be addressed.
... The advantage of this approach is that the FDA is not locked into making specific recommendations
... Regulatory Affairs for Biomaterials and Medical Devices that may appear reasonable at the time of publication but later on prove to be erroneous or irrelevant. [ IF A MEDICAL DEVICE - APPROVED BY THE FDA - KILLS AN AMERICAN - THEY ARE NOT TO BLAME - AND NEITHER IS THE "MANUFACTURER" ] ... The disadvantage from the point of view of the company is that they may make a submission only to find that the reviewers have a different understanding of the guidelines than they did. ... A complicating factor is that the specific contents of submissions and the review process are not in the public domain.
... There is no body of literature detailing the FDA review process with respect to specific products. [THUS] It is thus difficult to gage the chances of success with a particular strategy: as would be the case in common law, for example.
... To make matters worse, the FDA’s thinking also changes over time: the FDA is responsive to adverse events (which may be in the public domain), or issues being experienced by other companies who are submitting similar products (which are not in the public domain), and may require additional data and clarifications at any time. ...However, as mentioned previously, as clinical evidence emerges, the FDA may relax certain onerous criteria for approval. ... Thus, consultation with "experts" who closely follow these changes is crucial when developing a strategy for any given product. ... [ AND, HIRING FORMER FDA EMPLOYEES - AND EXPERTS ( WHO MAY USE YOUR PRODUCT) IS ADVISED ) ... at USA Jury trials ... demonstrations - of humans - being told to 'walk 12 feet AND sit down' - will be HELPFUL - TO CONVINCE THE "JUDGES" [THAT] NO ALL AMERICANS - FOLLOW "CLEAR" INSTRUCTIONS - THE SAME WAY ..." ]
https://www.odinhealthtech.com/#contact
https://www.imris.com/contact/
https://www.oncologysystems.com/contact-us ( DONE 6-9-2021 )
https://www.esaote.com/contacts/ ( DONE 6-9-2021 )
https://us.medical.canon/contact/ ( DONE 6-9-2021 )
HH HHH
HHH
https://www.usa.philips.com/healthcare/ ( DONE 6-9-2021 )
https://www.siemens-healthineers.com/en-us/how-can-we-help-you - ATTEMPT 6-9-2021
H 
https://www.gehealthcare.com/about/contact-us ( DONE 6-9-2021 )
MRI Systems Representative SALES > m.skok@PrizMedimaging.com
frank.shellock@mrisafety.com
MEDICAL Professors & Teachers
tkachjn@ucmail.uc.edu
david.hintenlang@osumc.edu
ugurb001@umn.edu
rmenon@robarts.ca
bhinfo@uw.edu
TOP-Of-Page
MEDICAL Publishers
AJNR.EIC@gmail.com
support@ispub.com
Research ScientistS - America
schepkin@magnet.fsu.edu
Research Facility - FRANCE
maryline.hevin@cea.fr
Academic Medical Centers
ivan.pedrosa@utsouthwestern.edu
robert.lenkinski@utsouthwestern.edu
neil.rofsky@utsouthwestern.edu
Ltsai1@bidmc.harvard.edu
akgrant@bidmc.harvard.edu
kmortele@bidmc.harvard.edu
jkung@bidmc.harvard.edu
shagen@bidmc.harvard.edu
Hospital - Technology Sites
drconnect@ccf.org
medicalconcierge@ccf.org
mvhssurgeryadministration@PremierHealth.com :: (DONE EMAILED) [ MARY Boosalis - PREMIER HEALTH-6-5-2021-PDF-VERSION.pdf cONTACT FORM ]
rearoberts@premierhealth.com (DONE EMAILED)
hrkirkpatr@PremierHealth.com (DONE EMAILED)
TOP-Of-Page
| Subject | Undeliverable: ACTION NEEDED- RE:FDA regulated "Medical Device" - fda-cdrh-cber-version |
| From | postmaster@fda.hhs.gov |
| To | REDACT |
| Date | Today 18:31 |
The email address you entered couldn't be found.
Please check the recipient's email address and try to resend the message. If the problem continues, please contact your email admin.
Diagnostic information for administrators: Generating server: FDSWV09481.fda.gov
shingchunbenny.lam@fda.hhs.gov
Remote Server returned '550 5.1.10 RESOLVER.ADR.RecipientNotFound; Recipient not found by SMTP address lookup'
Original message headers: Received: from FDSWV09481.fda.gov (10.168.16.247) by FDSWV09481.fda.gov (10.168.16.247) with Microsoft SMTP Server (version=TLS1_2, cipher=TLS_ECDHE_RSA_WITH_AES_256_CBC_SHA384_P256) id 15.1.2242.10; Mon, 7 Jun 2021 18:31:08 -0400 Received: from ironport7.fda.gov (10.168.18.7) by FDSWV09481.fda.gov (10.168.17.131) with Microsoft SMTP Server (version=TLS1_2, cipher=TLS_ECDHE_RSA_WITH_AES_256_CBC_SHA384_P256) id 15.1.2242.10 via Frontend Transport; Mon, 7 Jun 2021 18:31:08 -0400 IronPort-SDR: 6+v10PlWHHfs7CcYPnDG/Bu1H4XcQBTfdycnxBqqZF7qgOx4S4cqHQhxTsoTLD5MclpP/CV8cL gIliPLiRpG9419GOK4UwG4dl/C6seYCsk= X-SBRS: 2.5 X-MID: 115913618 IronPort-PHdr: ...
Reporting-MTA: dns;FDSWV09481.fda.gov
Received-From-MTA: dns;ironport7.fda.gov
Arrival-Date: Mon, 7 Jun 2021 22:31:08 +0000
Final-Recipient: rfc822;shingchunbenny.lam@fda.hhs.gov > Action: failed
Status: 5.1.10
Diagnostic-Code: smtp;550 5.1.10 RESOLVER.ADR.RecipientNotFound; Recipient not found by SMTP address lookup
| Subject | ACTION NEEDED- RE:FDA regulated "Medical Device" - fda-cdrh-cber-version |
| From | me TO FDA |
| Date | Today 18:21 |
6-7-2021 - a “MONDAY”
TO: Agents of United States FDA, CDRH, CBER ... MORE cONTENT - useful to cryptographers - BUT redacted (BY SUSAN (until needed) ...
( GROUP DONE )
jana.delfino@fda.hhs.gov
ting.song@fda.hhs.gov
sunder.rajan@fda.hhs.gov
shingchunbenny.lam@fda.hhs.gov << https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfstandards/detail.cfm?standard__identification_no=33222 :: eMAIL RETURNED BY FDA POSTMASTER 6-7-2021
( FDA Technical Contact - Shing Chun Benny Lam - FDA/OMPT/CDRH/OCD/ - 301-796-9328 - shingchunbenny.lam@fda.hhs.gov : [ NOW WITH PHILIPS ]
-- Standards Development Organization - IEEE Institute of Electrical and Electronic Engineers - https://www.ieee.orgFDA Specialty Task Group (STG) Radiology )
https://www.linkedin.com/in/shing-chun-benny-lam-120a5412
Philips Medical Systems :: https://www.usa.philips.com/healthcare/solutions/magnetic-resonance [ https://hansandcassady.org/Shing-Chun-Benny-Lam.html ]
wolfgang.kainz@fda.hhs.gov
Daniel.Krainak@fda.hhs.gov
combination@fda.gov
FDAImportsInquiry@fda.hhs.gov
DICE@fda.hhs.gov
reglist@cdrh.fda.gov
device.reg@fda.hhs.gov
TOP-Of-Page
( GROUP DONE )
CDRH Management - HHS/fda
jeff.shuren@fda.hhs.gov
Douglas.KellyMD@fda.hhs.gov
Ellen.Flannery@fda.hhs.gov
Elizabeth.Hillebrenner@fda.hhs.gov
Melissa.Torres@fda.hhs.gov
Nicole.Ellis@fda.hhs.gov
Nancy.Braier@fda.hhs.gov
Olga.Claudio@fda.hhs.gov
Cathy.Oliveri@fda.hhs.gov
Jerry.Menikoff@hhs.gov >>>>> done
Julia.Gorey@hhs.gov
douglas.diekema@seattlechildrens.org
ocrmail@hhs.gov
OCRMedia@hhs.gov [ PDF VERSION OF REQUEST ] via Jerry.Menikoff@hhs.gov (done)
( GROUP DONE )
Witten, Celia Center for Biologics Evaluation and Research -- ALSO REFERRED TO AS: CBER
Peter.Marks@fda.hhs.gov
email@asn-online.org
khi@asn-online.org
cberombudsman@fda.hhs.gov
TOP-Of-Page
Streamline Regulatory Support Service (example)
stephen.rhodes@streamlineregulatory.com
MORE DETAIL ON PERSONS MESSAGE SENT TO ( SEARCH VIA EMAIL )
MEDICAL DEVICE MANUFACTURERS
| rOW # | Company | Contact:NAME | CONTACT-Form | NOTES | ||||
| GE Healthcare | h | https://www.gehealthcare.com/about/contact-us | https://en.wikipedia.org/wiki/GE_Healthcare :: https://www.gehealthcare.com/products/bone-and-metabolic-health/lunar-idxa |
|||||
| Siemens Medical Systems | h | h | https://www.siemens-healthineers.com/en-us/how-can-we-help-you | https://www.siemens-healthineers.com/en-us/magnetic-resonance-imaging | ||||
| 3 | Philips Medical Systems | https://www.usa.philips.com/healthcare/medical-specialties/covid-19#_form | 3 | https://www.usa.philips.com/healthcare/solutions/magnetic-resonance | ||||
| 4 | Hitachi |
|
4 |
https://www.hitachihealthcare.com/ : https://en.wikipedia.org/wiki/Hitachi https://www.hitachi.co.in/social-innovation-business/healthcare-solution/magnet-resonance-imaging-system/?WT.ac=in_rm_socinn_healtsol_mris |
||||
| 5 |
"Toshiba Medical Systems" Canon Medical Systems Corporation |
https://us.medical.canon/contact/ | https://global.medical.canon/ | |||||
| 6 | Esaote S.p.A | https://www.esaote.com/contacts/ | https://www.esaote.com/en-US/contacts/ https://www.esaote.com/en-US/dedicated-mri/applications/neuro-imaging-head/ |
|||||
| 7 | ISOL Technology | https://www.oncologysystems.com/contact-us | https://www.oncologysystems.com/resources/mri-system-guides/isol-technologies-mri-systems | |||||
| 8 | Neusoft | brian.liuba@us.neusoft.com | http://www.neusoftmedical.com/en/ | |||||
| 9 | Fonar Corporation |
Email: info@fonar.com FONAR Sales: sales@fonar.com Human Resources: FonarHR@fonar.com Investor Relation: Investor@fonar.com |
9 | http://www.fonar.com/ : http://www.fonar.com/contact.html | ||||
| IMRIS : United States Corporate Headquarters
IMRIS, Deerfield Imaging Inc. |
Email: customersupport@imris.com | https://www.imris.com/contact/ | https://www.imris.com/ | |||||
| MagneVu | BANKRUPTCY: https://www.auntminnie.com/index.aspx?sec=ser&sub=def&pag=dis&ItemID=77843 | |||||||
| Odin Medical Technologies | https://www.odinhealthtech.com/#contact | "... Acquisition•Aug 1, 2006 Medtronic acquired Odin Medical Technologies for $9,000,000 ..." SOURCE: https://www.fdanews.com/articles/62028-medtronic-buys-odin-for-9m |
||||||
| ONI Medical Systems, Inc. | https://www.massdevice.com/ge-healthcare-completes-oni-medical-systems-buyout/ " GE Healthcare completes ONI Medical Systems buyout " |
|||||||
| Virgo - Product Millennium Technology |
https://www.diagnosticimaging.com/view/fda-clears-millenniums-virgo-open-mri https://www.accessdata.fda.gov/cdrh_docs/pdf/K990153.pdf https://www.milleniummri.com/ https://www.diagnosticimaging.com/view/canadian-start-firm-millennium-harnesses-low-cost-mri-technology |
MORE: MEDICAL Safety tRAINING
MRI Researchers AND SAFETY Agents
https://www.acr.org/-/media/ACR/Files/Radiology-Safety/MR-Safety/Manual-on-MR-Safety.pdf
"MRI" Frank G. Shellock "contact" frank.shellock@mrisafety.com : http://www.magneticresonancesafetytesting.com/Contact_Us.php : http://www.imrser.org/genpg.asp
"MRI" Jean A. Tkach "contact" : https://www.cincinnatichildrens.org/bio/t/jean-tkach : jean.tkach@cchmc.org
"MRI" Paul M. Ruggieri "contact"
"MRI" Thomas J. "Masaryk" "contact" a DrConnect account, visit eclevelandclinic.org or e-mail drconnect@ccf.org email: medicalconcierge@ccf.org
"MRI" Peter A. "Rasmussen" "contact" "email" "@" ::
American Journal of Neuroradiology March 2003, 24 (3) 463-471;
Ajnr Contact - Authors are encouraged to contact the Editor-in-Chief ( AJNR.EIC@gmail.com ) before preparing an unsolicited Review Article to avoid duplication of other work already in progress.May 1, 2021
Citation: Jennifer Jerrolds & Shane Keene:
TITLE: "MRI Safety at 3T versus 1.5T" :
The Internet Journal of World Health and Societal Politics. 2009; Volume 6, Number 1. support@ispub.com Internet Science Publications
https://www.acr.org/-/media/ACR/Files/Radiology-Safety/MR-Safety/Manual-on-MR-Safety.pdf
Funeral:Memorial : https://rauschfuneralhomes.com/service/loren-andrew-zaremba/ )
Loren A. Zaremba, Ph.D. :: ( https://www.ajronline.org/doi/full/10.2214/ajr.178.6.1781335?mobileUi=0 ;
https://www.nature.com/articles/d41586-018-07182-7
TOP-Of-Page
med.umn.edu/bio/department-of-neuroscience/kamil-ugurbil ugurb001@umn.edu
University of Minnesota’s Center for Magnetic Resonance Research, centre’s director, Kamil Ugurbil,
Ravi Menon, a neuroimaging scientist at Robarts Research Institute at Western University in London, Canada. rmenon@robarts.ca https://cfmm.uwo.ca/people/core_scientists/ravi_menon.html
says Victor Schepkin of the US National High Magnetic Field Laboratory in Tallahassee, Florida. schepkin@magnet.fsu.edu https://nationalmaglab.org/user-facilities/nmr-mri/all-nmr-mri-amris-staff/staff-nmr?view=personnel&id=VictorSchepkin
says Gregory Chang, a musculoskeletal radiologist at the New York University School of Medicine, .. doi.org/cwbr
https://nyulangone.org/doctors/1881892883/gregory-chang : https://journals.lww.com/topicsinmri/Citation/2019/06000/Preface.1.aspx#
http://www.ryanjbrown.com/contact-1
The NeuroSpin Centre in France Email: maryline.hevin@cea.fr https://www.meteoreservice.com/neurospin/ Specify ENGLISH
( https://prizmedimaging.com/ ) -- m.skok@PrizMedimaging.com :: MRI-EquipMent-Maker-copy-5-19-2021-11.JPG ... MANUFACTURERS INFORMATION FROM: Mike Skok : PrizMED Imaging Solutions - Expanded by Susan ... mri_Mike-Skok-SELLS-MRI-machines-01.JPG
TOP-Of-Page
Mara M. Barth, :: https://health.usnews.com/doctors/mara-kunst-661788
Martin P. Smith, ::::: https://findadoc.bidmc.org/details/1474/martin-smith-interventional_radiology_and_diagnostic_radiology-boston-milton ..."
Martin P. Smith < :: https://www.harringtonhospital.org/physician/smith-martin-p/ :: Email: shagen@bidmc.harvard.edu Susan hagan
Ivan Pedrosa, :: https://profiles.utsouthwestern.edu/profile/126193/ivan-pedrosa.html?&skip=140&max=10 ivan.pedrosa@utsouthwestern.edu
Robert E. Lenkinski, mailto: robert.lenkinski@utsouthwestern.edu
Neil M. Rofsky mailto: neil.rofsky@utsouthwestern.edu
Leo L. Tsai , : [ pubs.rsna.org/author/Tsai%2C+Leo+L ] :: https://findadoc.bidmc.org/details/1788/leo-tsai-diagnostic_radiology-boston-milton Leo Lee Tsai, M.D., Ph.D. > https://connects.catalyst.harvard.edu/Profiles/display/Person/54080
Title Assistant Professor of Radiology : Institution Beth Israel Deaconess Medical Center
Department Radiology : Beth Israel Deaconess Medical Center :: Email Ltsai1@bidmc.harvard.edu
Aaron K. Grant , : https://www.bidmc.org/research/research-by-department/radiology/mri-research/facilities/preclinical-mri-core ::
mailto: akgrant@bidmc.harvard.edu
TOP-Of-Page
Koenraad J. Mortele, Contact: kmortele@bidmc.harvard.edu : mailto: kmortele@partners.org https://www.advancedbodyimaging.org/Portals/9/Meetings/2010/MORTELE%20Rectal%20and%20perirectal%20diseases%20New%20imaging%20considerations.pdf
Justin W. Kung , : https://connects.catalyst.harvard.edu/Profiles/display/Person/34411 ::
Email jkung@bidmc.harvard.edu
TOP-Of-Page
Mary "Boosalis", president and CEO of "Premier Health" "@" [ MARY Boosalis - PREMIER HEALTH-6-5-2021-PDF-VERSION.pdf ]
FORM: https://www.premierhealth.com/locations/hospitals/miami-valley-hospital/contact-us < DONE
via email to: mvhssurgeryadministration@PremierHealth.com ..."
hospital purchasing > MRI [ Magnetic resonance imaging (MRI) ] :
Miami Valley North Hospital, Englewood, Ohio "Magnetic resonance imaging" (MRI) Purchasing
"Ohio" "Miami Valley" "Hospital" "North" "Magnetic resonance imaging"
- https://www.premierhealth.com/services/diagnostics-and-imaging/medical-imaging
Media Contact: Renee Roberts :: mobile 937-673-9376 :: rearoberts@premierhealth.com
PATIENT SAFETY AGENT: Kirkpatrick, Holly < hrkirkpatr@PremierHealth.com >
Susan's husband (of 39+ years) : Hans Neuhart : hans eig.net <hans@eig.net>
Victor Perry MD Neurosurgeon https://www.castleconnolly.com/top-doctors/victor-l-perry-neurological-surgery-56cc000036
https://www.samc.com/location/saint-agnes-medical-center?utm_campaign=website-link&utm_medium=organic&utm_source=local-listing
https://www.samc.com/site-search/search-results?q=Perry
- Victor Lynn Perry, MD
Medical Office
7255 N Cedar Ave #102 Fresno, California 93720
559-525-9088
also: 7887 N CEDAR Ave Fresno, CA 93720
Send To :
https://www.mizuho.co.jp/en/message/
To: Hiroshi Nemoto, Representative - Director - President & CEO, Mizuho Medical Co Japan & America
( https://www.mizuho.com/about-us ) :: mailto:%20 customerservice@mizuho.com
United States Food and Drug Administration :FDA "MRI" related pages : :: "contact" US FDA "alert" [ Thalidomide : https://en.wikipedia.org/wiki/Kefauver_Harris_Amendment ]
mailto: FDAImportsInquiry@fda.hhs.gov :: https://www.fda.gov/industry/import-program-food-and-drug-administration-fda
mailto: combination@fda.gov https://www.fda.gov/combination-products
https://www.fda.gov/medical-devices
SOURCE: https://www.fda.gov/medical-devices/medical-device-safety
"... Medical Device Safety ... The FDA monitors reports of adverse events and other problems with medical devices and alerts health professionals and the public when needed to ensure proper use of devices and the health and safety of patients. The lists below contain our most recent information. Other safety communications can be found using the links on the left side of this page. For additional information, contact us at: 1-800-638-2041 or DICE@fda.hhs.gov. ..."
https://search.usa.gov/search?query=mri&affiliate=fda1 :: RESULTS - QUERY = "MRI"
https://www.fda.gov/consumers/consumer-updates/fda-explores-new-uses-mri-scans
https://www.fda.gov/consumers/consumer-updates/fda-101-how-use-consumer-complaint-system-and-medwatch < Consumer Complaint System and MedWatch
[ https://www.fda.gov/safety/report-problem-fda/consumer-complaint-coordinators ] Ohio > telephone> 513- 679-2700 : 800-437-2382
[ SOURCE: https://www.fda.gov/safety/questions-and-answers-problem-reporting/what-happens-when-problem-reported "... National Tracking Systems ... Consumer product reports serve as an important alert system for FDA. Tracking systems for consumer reports are maintained in FDA's national headquarters. Reports from across the nation are forwarded to the appropriate headquarters offices whenever the problem involves a baby food, drug reaction, or any illness, injury, or life-threatening situation related to an FDA-regulated product. ..."
https://www.fda.gov/consumers/consumer-updates/how-report-product-problems-and-complaints-fda
https://www.fda.gov/consumers/consumer-updates/advisory-committees-give-fda-critical-advice-and-public-voice
https://search.usa.gov/search?query=magnetic+resonance+imaging&affiliate=fda1 :: RESULTS - QUERY = "magnetic resonance imaging"
https://www.fda.gov/radiation-emitting-products/medical-imaging/mri-magnetic-resonance-imaging
( https://www.fda.gov/medical-devices/cdrh-research-programs/electromagnetic-modeling < PAGE NOT FOUND 5-30-2021 )
https://www.fda.gov/medical-devices/guidance-documents-medical-devices-and-radiation-emitting-products/recent-final-medical-device-guidance-documents
"... Submit Comments -- Submit Comments Online ... You can submit online or written comments on any guidance at any time (see 21 CFR 10.115(g)(5)) > https://www.govinfo.gov/app/details/CFR-2012-title21-vol1/CFR-2012-title21-vol1-sec10-115
[ SOURCE: https://www.govinfo.gov/content/pkg/CFR-2013-title21-vol1/pdf/CFR-2013-title21-vol1-sec10-90.pdf "... (f) In unusual situations involving an immediate and significant danger to health, the Commissioner may take appropriate civil enforcement action contrary to an advisory opinion before amending or revoking the opinion. This action may be taken only with the approval of the Commissioner, who may not delegate this function. Appropriate amendment or revocation of the advisory opinion involved will be expedited. ..."
If unable to submit comments online, please mail written comments to: Dockets Management, Food and Drug Administration, 5630 Fishers Lane, Rm 1061, Rockville, MD 20852 ::
All written comments should be identified with this document's docket number: FDA-2019-D-2837. ..."
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/center-devices-and-radiological-health-cdrh-appeals-processes-questions-and-answers-about-517a
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/testing-and-labeling-medical-devices-safety-magnetic-resonance-mr-environment
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/magnetic-resonance-mr-receive-only-coil-performance-criteria-safety-and-performance-based-pathway
BY: Frank G. Shellock, Jean A. Tkach, Paul M. Ruggieri, Thomas J. Masaryk and Peter A. Rasmussen :: American Journal of Neuroradiology March 2003, 24 (3) 463-471;
Citation: Jennifer Jerrolds & Shane Keene: TITLE: "MRI Safety at 3T versus 1.5T" : The Internet Journal of World Health and Societal Politics. 2009; Volume 6, Number 1.
https://www.acr.org/-/media/ACR/Files/Radiology-Safety/MR-Safety/Manual-on-MR-Safety.pdf
Funeral:Memorial : https://rauschfuneralhomes.com/service/loren-andrew-zaremba/ )
Loren A. Zaremba, Ph.D. :: ( https://www.ajronline.org/doi/full/10.2214/ajr.178.6.1781335?mobileUi=0 ;
https://www.nature.com/articles/d41586-018-07182-7
med.umn.edu/bio/department-of-neuroscience/kamil-ugurbil
University of Minnesota’s Center for Magnetic Resonance Research, centre’s director, Kamil Ugurbil, had been waiting for years for this day. ays. [ Kamil Ugurbil ]
joliot.cea.fr/drf/joliot/Pages/Entites_de_recherche/NeuroSpin.aspx
Ravi Menon, a neuroimaging scientist at Robarts Research Institute at Western University in London, Canada.
says Victor Schepkin of the US National High Magnetic Field Laboratory in Tallahassee, Florida.
says Gregory Chang, a musculoskeletal radiologist at the New York University School of Medicine, .. doi.org/cwbr
Beyond 7 ... [ "Schepkin" US National High Magnetic Field Laboratory ]
he NeuroSpin Centre in France w
( https://prizmedimaging.com/ ) -- m.skok@PrizMedimaging.com :: MRI-EquipMent-Maker-copy-5-19-2021-11.JPG ... MANUFACTURERS INFORMATION FROM: Mike Skok : PrizMED Imaging Solutions - Expanded by Susan ... mri_Mike-Skok-SELLS-MRI-machines-01.JPG
Mara M. Barth, :: Dr. Mara M. Kunst (Barth) MD
https://health.usnews.com/doctors/mara-kunst-661788
Martin P. Smith, ::::: https://findadoc.bidmc.org/details/1474/martin-smith-interventional_radiology_and_diagnostic_radiology-boston-milton ..."
Ivan Pedrosa, ::::: https://profiles.utsouthwestern.edu/profile/126193/ivan-pedrosa.html?&skip=140&max=10
Robert E. Lenkinski :: https://www.utsouthwestern.edu/facultydata/126382/files/Lenkinski%20CV%20123115.pdf
Neil M. Rofsky :: https://utsouthwestern.pure.elsevier.com/en/persons/neil-m-rofsky
Leo L. Tsai , : [ pubs.rsna.org/author/Tsai%2C+Leo+L ] :: https://findadoc.bidmc.org/details/1788/leo-tsai-diagnostic_radiology-boston-milton
Aaron K. Grant, : https://www.bidmc.org/research/research-by-department/radiology/mri-research/facilities/preclinical-mri-core ::
Koenraad J. Mortele, : https://www.advancedbodyimaging.org/Portals/9/Meetings/2010/MORTELE%20Rectal%20and%20perirectal%20diseases%20New%20imaging%20considerations.pdf :: kmortele@partners.org
Justin W. Kung, : https://connects.catalyst.harvard.edu/Profiles/display/Person/34411 ::
Martin P. Smith < :: https://www.harringtonhospital.org/physician/smith-martin-p/
Mary "Boosalis", president and CEO of "Premier Health" "@"
via email to: mvhssurgeryadministration@PremierHealth.com ..." "@PremierHealth.com" : @ "PremierHealth.com"
Miami Valley North Hospital, Englewood, Ohio "Magnetic resonance imaging" (MRI) Purchasing
- https://www.ovsurgical.com/contact-us.html?gclid=EAIaIQobChMIkJzG2s7V8AIVdB6tBh0PswvGEAAYASABEgJjX_D_BwE
- https://www.premierhealth.com/locations/hospitals/miami-valley-hospital-north/contact-us : FORM
"Ohio" "Miami Valley" "Hospital" "North" "Magnetic resonance imaging"
- https://www.premierhealth.com/services/diagnostics-and-imaging/medical-imaging
Media Contact: Renee Roberts :: mobile 937-673-9376 :: rearoberts@premierhealth.com
From: Kirkpatrick, Holly <hrkirkpatr@PremierHealth.com>
TOP-Of-Page
FDA - REGULATED MEDICAL DEVICEs
: Recognized Consensus Standard :
GENERAL
| Device | System, Nuclear Magnetic Resonance Imaging |
|---|---|
| Regulation Description | Magnetic resonance diagnostic device. |
| Regulation Medical Specialty | Radiology |
| Review Panel | Radiology |
| Product Code | LNH |
| Premarket Review | Division of Radiological Health (DRH) Division of Radiological Health (DRH) |
| Submission Type | 510(k) |
| Regulation Number | 892.1000 |
| Device Class | 2 |
| Total Product Life Cycle (TPLC) | TPLC Product Code Report |
FDA CONTINUED...
| H |
Product Classification |
Supplementary Information Sheet (SIS) | PUBLIC LAW : fda ENFORCEMENT AGENT | H | |||
| 12-187 NEMA MS 3-2008 (R2020) | https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfStandards/detail.cfm?standard__identification_no=33531 | Jana Delfino | jana.delfino@fda.hhs.gov | ||||
| H | 12-188 NEMA MS-1-2008 (R2020) | https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfStandards/detail.cfm?standard__identification_no=33530 | Ting Song | ting.song@fda.hhs.gov | H | ||
| "" | "" | Sunder Rajan | sunder.rajan@fda.hhs.gov | ||||
| 12-195 NEMA MS 6-2008 (R2014) | https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfStandards/detail.cfm?standard__identification_no=33530 | Sunder Rajan | sunder.rajan@fda.hhs.gov | ||||
| https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfstandards/detail.cfm?standard__identification_no=33222 | Shing Chun Benny Lam | shingchunbenny.lam@fda.hhs.gov | |||||
| www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfStandards/detail.cfm?standard__identification_no=37168 | Wolfgang Kainz | wolfgang.kainz@fda.hhs.gov | |||||
| https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfStandards/detail.cfm?standard__identification_no=34029 | Daniel Krainak | Daniel.Krainak@fda.hhs.gov | |||||
| https://www.fda.gov/combination-products | combination@fda.gov | ||||||
| https://www.fda.gov/industry/import-program-food-and-drug-administration-fda/import-basics | FDAImportsInquiry@fda.hhs.gov | ||||||
| https://www.fda.gov/about-fda/cdrh-offices/division-industry-and-consumer-education | DICE@fda.hhs.gov | ||||||
| https://www.fda.gov/medical-devices/how-study-and-market-your-device/device-registration-and-listing | reglist@cdrh.fda.gov | ||||||
| https://www.fda.gov/training-and-continuing-education/cdrh-learn | device.reg@fda.hhs.gov | ||||||
TOP-Of-Page
12-187 NEMA MS 3-2008 (R2020) :: Determination of Image Uniformity in Diagnostic Magnetic Resonance Images
Jana Delfino
FDA/OC/CDRH/OPEQ/OIDRH/DRH/MREPB/
301-796-6503
jana.delfino@fda.hhs.gov
12-188 NEMA MS-1-2008 (R2020) :: Determination of Signal-to-Noise Ratio (SNR) in Diagnostic Magnetic Resonance Imaging
Ting Song
FDA/OC/CDRH/OPEQ/OHTVI/DHTVIA/
301-796-7677
ting.song@fda.hhs.gov
Sunder Rajan
FDA/OC/CDRH/OSEL/DBP/
301-796-4194
sunder.rajan@fda.hhs.gov
12-195 NEMA MS 6-2008 (R2014)
Determination of Signal-to-Noise Ratio and Image Uniformity for Single-Channel Non-Volume Coils in Diagnostic MR Imaging
FDA Technical Contacts
Sunder Rajan
FDA/OC/CDRH/OSEL/DBP/
301-796-4194
sunder.rajan@fda.hhs.gov
Jana Delfino
FDA/OC/CDRH/OPEQ/OIDRH/DRH/MREPB/
301-796-6503
jana.delfino@fda.hhs.gov
Standards Development Organization
12-196 NEMA MS 2-2008 (R2020)
Determination of Two-Dimensional Geometric Distortion in Diagnostic Magnetic Resonance Images
FDA Technical Contacts
Ting Song
FDA/OC/CDRH/OPEQ/OHTVI/DHTVIA/
301-796-7677
ting.song@fda.hhs.gov
Sunder Rajan
FDA/OC/CDRH/OSEL/DBP/
301-796-4194
sunder.rajan@fda.hhs.gov
TOP-Of-Page
12-232 NEMA MS 4-2010
Acoustic Noise Measurement Procedure for Diagnosing Magnetic Resonance Imaging Devices
FDA Technical Contact
Ting Song
FDA/OC/CDRH/OPEQ/OHTVI/DHTVIA/
301-796-7677
ting.song@fda.hhs.gov
12-264 NEMA MS 11-2010
Determination of Gradient-Induced Electric Fields in Diagnostic Magnetic Resonance Imaging
FDA Technical Contact
Ting Song
FDA/OC/CDRH/OPEQ/OHTVI/DHTVIA/
301-796-7677
ting.song@fda.hhs.gov
12-288 NEMA MS 9-2008 (R2020)
Standards Publication Characterization of Phased Array Coils for Diagnostic Magnetic Resonance Images
FDA Technical Contact
Jana Delfino
FDA/OC/CDRH/OPEQ/OIDRH/DRH/MREPB/
301-796-6503
jana.delfino@fda.hhs.gov
12-292 IEEE Std 3333.2.1-2015
IEEE Recommended Practice for Three-Dimensional (3D) Medical Modeling
TOP-Of-Page
FDA Technical Contact
Shing Chun Benny Lam
FDA/OMPT/CDRH/OCD/
301-796-9328
shingchunbenny.lam@fda.hhs.gov < RETURNED BY FDA POSTMASTER 6-7-2021
12-295 IEC 60601-2-33 Ed. 3.2 b:2015
Medical electrical equipment - Part 2-33: Particular requirements for the basic safety and essential performance of magnetic resonance equipment for medical diagnosisFDA Technical Contacts
Wolfgang Kainz
FDA/OC/CDRH/OSEL/DBP/
301-796-7595
wolfgang.kainz@fda.hhs.gov
Jana Delfino
FDA/OC/CDRH/OPEQ/OIDRH/DRH/MREPB/
301-796-6503
jana.delfino@fda.hhs.gov
12-298 NEMA MS 10-2010
Determination of Local Specific Absorption Rate (SAR) in Diagnostic Magnetic Resonance Imaging
FDA Technical Contact
Ting Song
FDA/OC/CDRH/OPEQ/OHTVI/DHTVIA/
301-796-7677
ting.song@fda.hhs.gov
12-306 NEMA MS 12-2016
Quantification and Mapping of Geometric Distortion for Special Applications
FDA Technical Contact
Ting Song
FDA/OC/CDRH/OPEQ/OHTVI/DHTVIA/
301-796-7677
ting.song@fda.hhs.gov
TOP-Of-Page
12-315 NEMA MS 8-2016
Characterization of the Specific Absorption Rate (SAR) for Magnetic Resonance Imaging Systems
FDA Technical Contacts
Wolfgang Kainz
FDA/OC/CDRH/OSEL/DBP/
301-796-7595
wolfgang.kainz@fda.hhs.gov
Ting Song
FDA/OC/CDRH/OPEQ/OHTVI/DHTVIA/
301-796-7677
ting.song@fda.hhs.gov
Jana Delfino
FDA/OC/CDRH/OPEQ/OIDRH/DRH/MREPB/
301-796-6503
jana.delfino@fda.hhs.gov
12-322 NEMA MS 5-2018
Determination of Slice Thickness in Diagnostic Magnetic Resonance Imaging
FDA Technical Contact
Ting Song
FDA/OC/CDRH/OPEQ/OHTVI/DHTVIA/
301-796-7677
ting.song@fda.hhs.gov
12-331 NEMA Standards Publication MS 14-2019
Characterization of Radiofrequency (RF) Coil Heating in Magnetic Resonance Imaging Systems
FDA Technical Contact
Daniel Krainak
FDA/OC/CDRH/OPEQ/OIDRH/DRH/MREPB/
301-796-0478
Daniel.Krainak@fda.hhs.gov
FDA related > CDRH Management Directory by Organization
| hhhhhhhhh | |||
| Center Director | Jeffrey Shuren, M.D., J.D. | 301-796-5900 | jeff.shuren@fda.hhs.gov |
| Deputy Center Director for Science | Douglas Kelly, M.D. | 301-796-5900 | Douglas.KellyMD@fda.hhs.gov |
| Deputy Center Director for Policy | Ellen Flannery, J.D. | 301-796-5900 | Ellen.Flannery@fda.hhs.gov |
| Associate Director for Scientific and Regulatory Programs | Elizabeth Hillebrenner | 301-796-5900 | Elizabeth.Hillebrenner@fda.hhs.gov |
| Associate Director for International Affairs | Melissa Torres | 301-796-5576 | Melissa.Torres@fda.hhs.gov |
| Associate Director for Strategic Communications | Nicole Ellis | 301-796-5900 | Nicole.Ellis@fda.hhs.gov |
| Quality Management Staff | |||
| Staff Director and Associate Director for Quality Management | Nancy Collazo-Braier, Ph.D. | 301-796-5676 | Nancy.Braier@fda.hhs.gov |
| Assistant Director for Quality Management Systems | Olga Claudio | 301-796-7601 | Olga.Claudio@fda.hhs.gov |
| Assistant Director for Process Improvement | Cathy Oliveri | 301-796-5549 | Cathy.Oliveri@fda.hhs.gov |
US Health and Human Services : The Secretary of Health and Human Services >
"Xavier Becerra" "contact" : https://www.hhs.gov/about/contact-us/index.html Email: ocrmail@hhs.gov emailing OCRMedia@hhs.gov
https://www.hhs.gov/about/contact-us/index.html : Douglas S. Diekema, M.D., M.P.H. - Chair, Secretary’s Advisory - Committee on Human Research Protections (SACHRP) - Email: bhinfo@uw.edu - douglas.diekema@seattlechildrens.org
Jerry Menikoff, M.D., J.D., Executive Secretary, SACHRP Jerry.Menikoff@hhs.gov
Julia Gorey, J.D., Executive Director, SACHRP Julia.Gorey@hhs.gov : "... SACHRP Committee ...The Secretary’s Advisory Committee on Human Research Protections (SACHRP) provides expert advice and recommendations to the Secretary of HHS on issues pertaining to the protection of human subjects in research. ..."
https://www.fda.gov/about-fda/what-we-do : FDA Mission "... The Food and Drug Administration is responsible for protecting the public health by ensuring the safety, efficacy, and security of ... medical devices ..."
https://www.fda.gov/about-fda/fda-organization "... FDA is an agency within the Department of Health and Human Services. ..."
https://www.fda.gov/about-fda/fda-organization/jeffrey-shuren : jeff.shuren@fda.hhs.gov
https://www.fda.gov/about-fda/fda-organization/center-devices-and-radiological-health
US Health and Human Services - https://www.hhs.gov/about/index.html :: MISSION: "... The mission of the U.S. Department of Health and Human Services (HHS) is to enhance the health and well-being of all Americans, by providing for effective health and human services and by fostering sound, sustained advances in the sciences underlying medicine, public health, and social services. ..."
https://www.accessdata.fda.gov/scripts/medwatch/index.cfm
https://www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=consumer.reporting1
https://www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=consumer.reporting6
https://www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=reporting.confirmation
MedWatch Voluntary Report ( PAGE COPY ) report made 6-3-2021
| What kind of problem was it? |
|
|---|---|
| Did any of the following happen? |
|
| Date the problem occurred: | |
| Tell us what happened and how it happened: | 5-18-2021 cONSUMER - WITH INSTALLED ANEURYSM CLIP ARRIVED FOR MRI-MRA DIAGNOSTIC SCAN cLIP APPROVED FOR 1.5 TESLA POWER - SCHEDULED DEVICE WAS 3.0 TESLA |
| Relevant Tests/Laboratory Data: |
|
| Additional Comments: | |
| Please select the cause of the problem that applies below: |
|
| Do you still have the product in case we need to evaluate it? | Yes |
| Do you have a picture of the product? | Yes |
| Images attached: | BRAIN-IMPLANT-INFORMATION-PAGE.JPG |
| Name of medical device: | MRI/MRA 3.0 TESLA IMAGING DEVICE |
|---|---|
| Name of the company that makes the medical device: | GE HEALTHCARE & OTHERS |
| Model number: | |
| Catalog number: | |
| Lot number: | |
| Serial number: | |
| UDI number: | |
| Expiration Date: | |
| Was someone operating the medical device when the problem occurred? | No |
| If yes, who was operating it? | |
| Date the implant was put in: | 12/18/2012 |
| Date the implant was taken out: |
| Person's Initials: | S N |
|---|---|
| Gender: | Female |
| Age: | |
| Date of Birth: | 05/10/1954 |
| Weight: | |
| Ethnicity: | Not Hispanic/Latino |
| Race: | White |
| List known medical conditions: | https://hansandcassady.org/Brain-Implant-information.html < WEB PAGE DESCRIBING |
| Please list all allergies: | NA |
| List any other important information about the... | IS RETIRED SOFTWARE ENGINEERING TECHNICAL WRITER - CAPABLE OF CREATING WEB PAGES : WELL EDUCATED [UNIVERSITY OF WISCONSIN) TO "RAISE ARMY" - IF NEEDED. PREFERS TO WRITE LETTERS TO NEWSPAPER EDITOR - SUPPORTED KAMALA HARRIS - WILL WORK WITH BIDEN & Xavier Becerra https://hansandcassady.org/SEND-TO-MRI-SAFETY.html |
| List all current prescription medications and medical devices being used: | NA - PERFORMS YOGA POSES |
| List all over-the-counter medications and any vitamins, minerals, supplements, and herbal remedies being used: | ALEVE VITAMINS |
| Name: | NEUHART (CASSADY), Susan |
|---|---|
| Preferred Address: | redact |
| Telephone number: | |
| Email address: | redact |
| Did you report this problem to the company that makes the product (the manufacturer/compounder)? | No |
MedWatch Voluntary Report
Thank you for submitting your report to MedWatch, the FDA Safety Information and Adverse Event Reporting Program.
This acknowledgement confirms that your report was received. Reports are added to FDA's post marketing safety database with similar reports and reviewed by the FDA's medical products safety staff.
Voluntary reports are essential for ensuring the continued safety of FDA-regulated products. One or two well-documented case reports may provide an early signal of unexpected problems and lead to additional evaluation. This may result in FDA regulatory actions that improve the safety of the products used in patient care each day.
Although we will not be able to provide you with details about the evaluation of your report, we will contact you if additional information is needed.
Again, thank you for taking the time to submit your report.
You will receive an email confirmation about your report from druginfo@fda.hhs.gov so please allow this email address if you have a spam blocker.
If you would like additional information here is our contact information.
| Product Type | E-mail Inquiries | Telephone Inquiries |
|---|---|---|
| Drugs | Drug Information Questions and Comments Email: druginfo@fda.hhs.gov |
Division of Drug Information (855) 543-3784 (301) 796-3400 |
| Special Nutritional Products (dietary supplements, infant formulas, medical foods), Cosmetics and Foods/Beverages | Food, Nutrition, and Cosmetics Questions & Answers Email: Consumer@fda.gov |
888-723-3366 |
| Medical Devices | Center for Devices and Radiological Health, Office of Communication and Education, Division of Industry and Consumer Education E-mail: dice@fda.hhs.gov |
1(800) 638-2041 or (301) 796-7100 |
| Vaccines, blood products, other biologics | Center for Biologics Evaluation and Research, Division of Communication and Consumer Affairs, Consumer Affairs Branch, Questions and Answers Email: ocod@fda.hhs.gov |
800-835-4709 |
U.S. Food and Drug Administration
10903 New Hampshire Avenue
Silver Spring, MD 20993
1-888-INFO-FDA (1-888-463-6332)
Center for Biologics Evaluation and Research -- ALSO REFERRED TO AS: CBER
Celia M. Witten > PERSON WHO SIGNED FDA APPROVAL LETTER - FOR ANEURYSM CLIP 1999 ( PDF )
"Deputy Director" : https://www.fda.gov/about-fda/center-biologics-evaluation-and-research-cber/center-biologics-evaluation-and-research
Peter W. Marks, MD, PhD : " Director" :: Peter.Marks@fda.hhs.gov
h 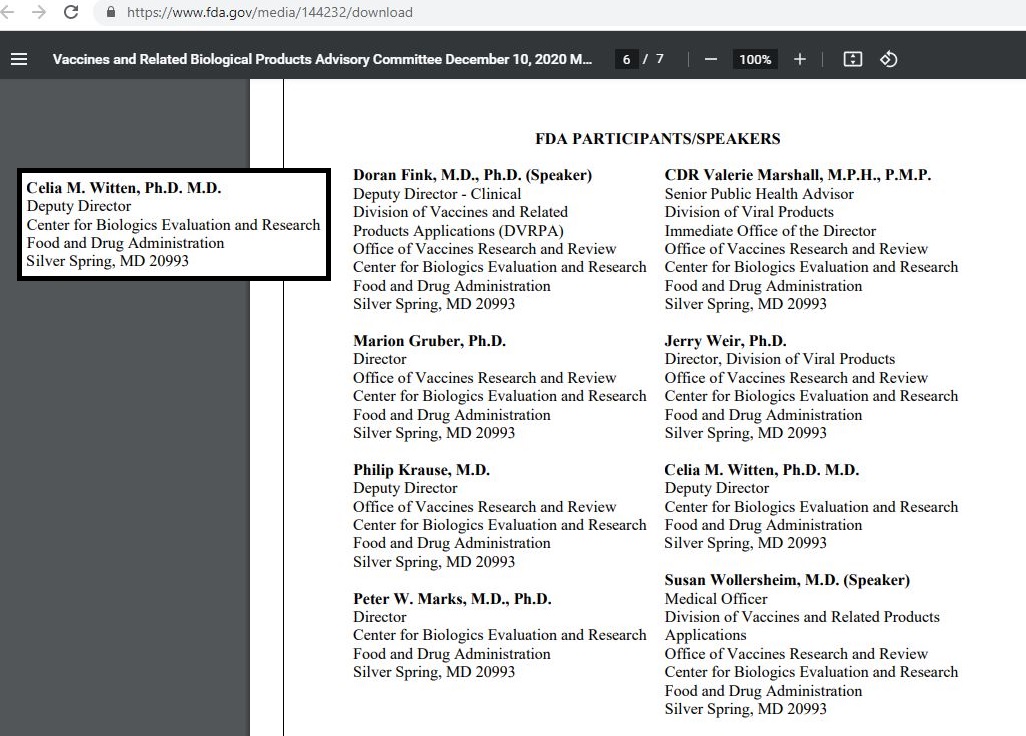
Celia M. Witten > PERSON WHO SIGNED FDA APPROVAL LETTER - FOR ANEURYSM CLIP 1999 ( PDF )
RELATED ARTICLE > https://www.ahajournals.org/doi/10.1161/01.STR.0000153063.54972.91
" The Food and Drug Administration Medical Device Review Process "
Celia M. Witten :: https://www.who.int/biologicals/expert_committee/WITTEN_Celia_BIO.pdf
https://slideplayer.com/slide/1594020/ < Witten
email@asn-online.org :: khi@asn-online.org
https://www.asn-online.org/about/bio.aspx?ID=3153784&title=KHI+Board+of+Directors
https://sites.utexas.edu/cpe-icdd/speakers/celia-witten/
https://www.fda.gov/media/144232/download
Celia M. Witten, Ph.D., M.D. :: Director, Division of General, Restorative and Neurological Devices < gOOGLE
Office of Device Evaluation : Center for Devices and Radiological Health
( https://www.fda.gov/about-fda/center-devices-and-radiological-health/reorganization-center-devices-and-radiological-health ) > " Reorganization of The Center for Devices and Radiological Health "
Deputy Director - Center for Biologics Evaluation and Research - Food and Drug Administration Silver Spring, MD 20993
General E-mail: cberombudsman@fda.hhs.gov
14 - Streamline Regulatory Support Service (example)
Email: stephen.rhodes@streamlineregulatory.com
https://en.wikipedia.org/wiki/Veeva_Systems
https://www.veeva.com/blog/four-key-areas-to-streamline-regulatory-operations-and-compliance/
SOURCE: https://www.streamlineregulatory.com/about
"... Streamline Regulatory offers a variety of services, including regulatory support on devices and combination products, nonclinical and clinical study design and evaluation, and litigation expert witness testimony.
... Streamline Regulatory offers:
Regulatory support to clients on devices and combination products
510(k)s, IDEs, Pre-Submissions, PMAs, 513(g)s, HDEs, and Requests for Designations submissions
Nonclinical and clinical study design and evaluation
Regulatory strategy documents
FDA meetings and interactions
Due diligence evaluations
Litigation expert witness testimony
... Stephen P. Rhodes, MS, Principal, "Streamline Regulatory"
Mr. Rhodes has 29 years of experience in the regulation of medical devices and combination products.
Prior to starting Streamline Regulatory in 2018, Mr. Rhodes worked as a Senior Consultant at Biologics Consulting Group for 8 years. Before joining BCG, Mr. Rhodes worked for more than 20 years at the Food and Drug Administration, Center for Devices and Radiological Health, Office of Device Evaluation:
- Director, Investigational Device Exemption (IDE) and Humanitarian Device Exemption (HDE) Programs (2007–2010)
- Acting Deputy Division Director, Division of General, Restorative, and Neurological Devices (2005)
- Acting Deputy Division Director, Division of General, Restorative, and Neurological Devices (2002)
- Acting Deputy Division Director, Division of Cardiovascular and Respiratory Devices (2001)
- Branch Chief, Plastic and Reconstructive Surgery Devices Branch (1996–2007)
- Team Leader, Orthopedic Devices Branch (1995)
- Engineering Reviewer, Orthopedic Devices Branch (1989–1996) ..."
[ END] TOP-Of-Page